Supplier Quality I
By: Pablo J Lebed
Published: August 14, 2025

As a laboratory professional, you play a critical role in your organization’s supplier quality dimension, often interacting directly with suppliers to ensure consistency and reliability. Understanding your supplier’s test methods is key to aligning expectations and ensuring quality. While the ideal scenario is for both you and your supplier to use the same test method, reality often deviates from this. Here’s how to navigate these challenges effectively.
Understanding Your Supplier’s Test Method
The first step in supplier quality management is gaining clarity on the test methods your supplier uses. This information is critical but not always readily available. Your Supplier Quality team may lack the technical expertise to fully understand these methods or know what specific questions to ask. As a laboratory professional, you’ll often step into this role.
Building a trusting relationship with your supplier takes time. Depending on the stage of your partnership, the supplier may share their test method in varying levels of detail. It’s essential to document all shared information—and any gaps—while clearly communicating the analytical chemistry implications to your Supplier Quality team.
A Real-World Example
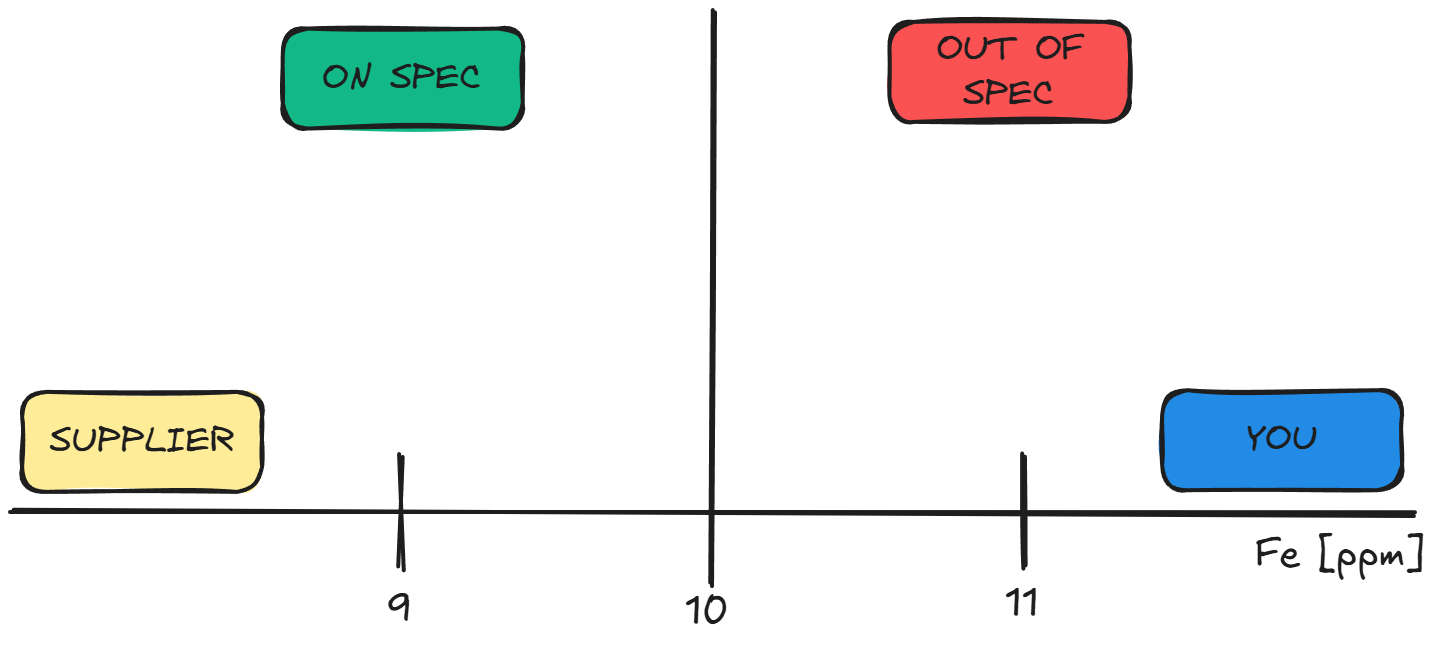
Consider this scenario: Your supplier provides a batch of material with a certificate of analysis stating that iron (Fe), an impurity, is within specification at less than 10 ppm. The Supplier Quality team takes a sample and requests analysis for iron using ICP-OES. Your lab’s analysis yields a result of 11 ppm, while the supplier’s certificate reports 9 ppm. Based on your results, the material appears out of specification.
This discrepancy highlights the importance of understanding the supplier’s test method. Differences in equipment, calibration, or procedural details could explain the variation. For instance:
- Did the supplier use a different analytical technique (e.g., ICP-MS vs. ICP-OES)?
- Were their calibration standards prepared differently?
- Could sample preparation or matrix effects have influenced the results?
Without clarity on the supplier’s method, resolving such discrepancies becomes challenging. Documenting these differences and discussing them with your Supplier Quality team is critical to making informed decisions.
Key Steps for Laboratory Professionals
- Request and Document Test Methods: Ask your supplier for detailed information about their analytical methods. Document everything, including any missing details, to track potential sources of variability.
- Assess Analytical Implications: Evaluate how differences in test methods might affect results. For example, variations in detection limits or matrix interferences can lead to discrepancies like the one in the example above.
- Collaborate with Supplier Quality Teams: Translate technical findings into actionable insights for non-technical team members to facilitate discussions with suppliers.
- Build Trust Over Time: As your relationship with the supplier develops, encourage open sharing of technical details to align methods and improve consistency.
In Short
Your role as a laboratory professional extends beyond running tests—it’s about bridging the gap between your organization and its suppliers. By understanding and documenting supplier test methods, you ensure better alignment, reduce discrepancies, and strengthen quality assurance processes. Invest time in building trust with suppliers and communicating technical insights to your team for better decision-making.